Pipeline
KalVista is advancing a pipeline of novel oral, small molecule medicines for diseases in which the Kallikrein-Kinin System (KKS) plays a key role, with an initial focus on hereditary angioedema (HAE).
Our oral small molecules, including protease inhibitors, are designed to intervene at key points in the KKS and have shown efficacy and safety in preclinical and clinical trials, reflecting our ability to precisely target the KKS pathway.
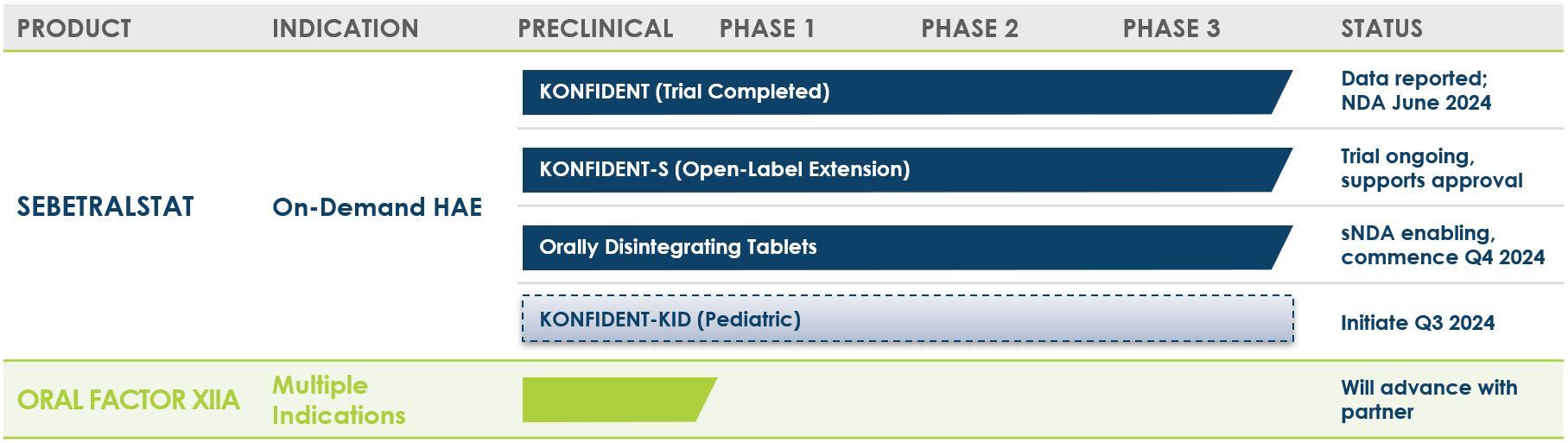
Sebetralstat for HAE
Sebetralstat is an oral plasma kallikrein inhibitor and the most advanced compound in our portfolio of candidates for the treatment of hereditary angioedema (HAE). Early treatment of HAE attacks has been shown to be key in maximizing treatment efficacy. Dosing with approved injectable treatments is often delayed undermining treatment outcomes. As a result of its straightforward oral administration combined with its rapid uptake and encouraging safety profile, sebetralstat has the potential to offer HAE patients an option to treat attacks at the earliest stages, before the symptoms develop more fully.
KalVista reported positive topline data from the Phase 3 KONFIDENT trial in February 2024, and, we expect to be able to file a New Drug Application (NDA) with the U.S. FDA in the first half of 2024. More information on sebetralstat and clinical results can be found here.
Factor XIIa
Our oral small molecule Factor XIIa (FXIIa) inhibitor program has the potential to be a next generation therapy for HAE prophylaxis with an efficacy, safety, and tolerability profile similar to injectable treatments. There is strong scientific rationale and positive clinical evidence for FXIIa inhibition in HAE prophylaxis. Clinical studies of a Factor XIIa antibody have demonstrated efficacy in reducing HAE attack frequency.(1)
While injectable Factor XIIa antibody therapies are currently in clinical studies for HAE prophylaxis and other indications, developing oral Factor XIIa inhibitors has been a significant scientific challenge, and our newly announced program represents a major breakthrough in this area. Our internal research team has discovered multiple series of low nanomolar potency Factor XIIa inhibitors with high degrees of selectivity and oral bioavailability. We are pursuing comprehensive IP protection for this advanced medicinal chemistry program that is currently in lead optimization.
(1) Craig TJ, Reshef A, Li HH, Jacobs JS, Bernstein JA, Farkas H, Yang WH, Stroes ESG, Ohsawa I, Tachdjian R, Manning ME, Lumry WR, Saguer IM, Aygören-Pürsün E, Ritchie B, Sussman GL, Anderson J, Kawahata K, Suzuki Y, Staubach P, Treudler R, Feuersenger H, Glassman F, Jacobs I, Magerl M. Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 401:1079-1090. doi: 10.1016/S0140-6736(23)00350-1.